Research / Registries
Cloud-based, multi-site clinical trial administration and patient access
Connecting the dots
between patients and research
Visiontree for research & registries is a 21 CFR Part 11 compliant cloud-based, interoperable application for multi-site clinical trial management and patient access.
The Visiontree application provides a library of over 400 electronic patient and clinician reported outcomes assessments for ePRO and eCOA use. Visiontree allows you to manage decentralized clinical trials to bridge the gap between clinical research and clinical care.
Proven to deliver a high level of patient compliance, accuracy and validation, Visiontree provides data collection and curation with EHR interoperablity in semantic context of the patient health record for optimal multi-center research & registry management and reporting.
Outcomes Packages by Specialty
Visiontree provides standards-based ePRO, eCOA and customized solutions in these, and other, core domains.
Patient & Clinical Outcomes Data Collection & Reporting
Nimble and interoperable. The Visiontree Optimal Care™ (VTOC) cloud-based system allows providers to deliver improved quality and efficiency for patient care.
- Efficient
Save time with cloud-based, paperless workflow for all clinical research and patient reported outcomes forms
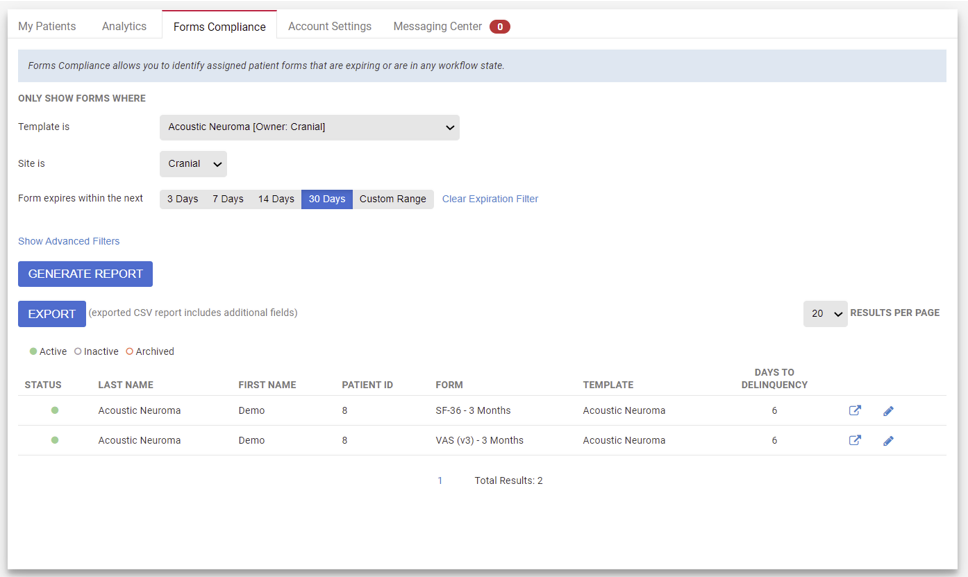
Form compliance alerts for patients and research teams
Access to an extensive library (1,000+) of electronic, validated electronic patient reported outcomes assessments (ePRO)
Template and Timepoint Manager to build and manage your own research protocols
- Accurate
Electronic patient reported outcomes data collection and reporting at specific timepoints
Research team and patient reminders by timepoint for high completion rates
Proven to increase patient reported outcomes data collection rate by 40%+ over paper
Template Manager allows for patients on multiple protocols, including separate treatment arms
- Interoperable
Integration with legacy EHR and research systems including Medidata Rave®
Import Hub for data mapping of historical data and scheduled exports from EHR and disparate databases
Connectivity with all data interchange standards (HL7, Web services)
Multi-institutional participation with site-level access and Admin-level Aggregate data reporting
Interested?
For more information.